BACKGROUND
Multiple myeloma is a largely incurable disease characterized by periods of response followed by relapse and/or refractoriness to therapy. With many new treatments available, patients now have more flexibility to assess treatment benefits and risks and what aspects of a treatment are most important to them. The purpose of this non-interventional qualitative study was to examine treatment-related decision-making in patients with MM and care partners with varying experience with refractoriness.
METHODS
Patients with MM and care partners were recruited through the Gryt Health Cancer Community, social media, and participant referrals to join a qualitative study centered around a semi-structured interview. Adult patients with MM who self-reported treatment for MM in the United States were eligible; adults who self-reported being the primary care partner to an adult with a MM diagnosis who had received treatment in the United States were also eligible. Participants were stratified based on refractoriness to at least one proteasome inhibitor, immunomodulatory drug, and/or anti-CD38 monoclonal antibody (non-refractory; single or double refractory; triple refractory). An Institutional Review Board approved this study.
RESULTS
The study was completed by 42 participants, of which 32 were patients with MM (12 non-refractory; 10 single or double refractory; 10 triple refractory) and 10 were MM care partners (3 non-refractory; 3 single or double refractory; 4 triple refractory) (Table). Each care partner was paired to a patient participant, and 80% lived in the same household as the patient. All care partners had provided support since the MM diagnosis, if not before, and at minimum had been providing support for at least 2 years. More than half (60%) of care partners were currently working full-time, relative to only 20% of the paired patients.
Patients and care partners made comments supportive of MM being approached as a chronic disease, which suggests an abrupt shift in perspective compared to historical sentiment. Participants wanted to retain or improve their quality of life (QoL) and focus less on the disease itself. Reasons for this shift in perspective include an increased sense of hope brought about by new treatments and community support, documented improvements in long-term survival, and better techniques for monitoring disease relapse, particularly minimal residual disease (MRD) status.
More than 70% of participants expressed that treatment-related decision-making was shared, particularly as they were further from diagnosis, and felt comfortable asking their doctor(s) questions related to treatment options, even if treatment options were only discussed on an as-needed basis. Care partners were more actively involved in decision-making around significant events, such as diagnosis, stem cell transplant, and relapses.
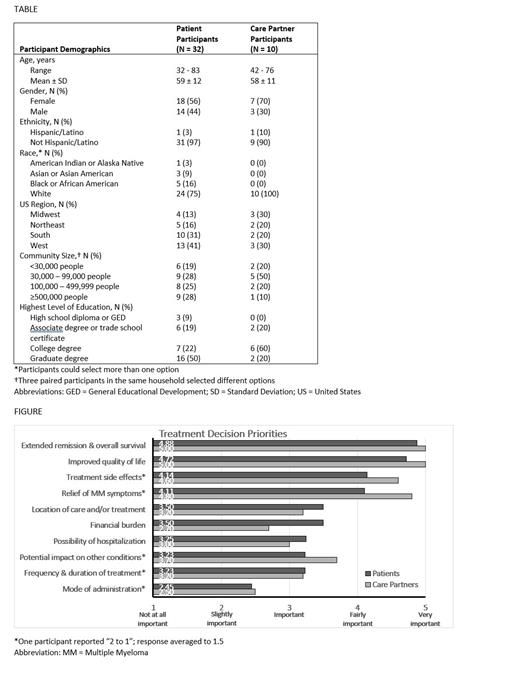
When making treatment decisions, participants were most concerned about extending remission and overall survival (OS), improving QoL, and minimizing MM symptoms and treatment-related side effects (Figure). Extending remission and OS and improving QoL were rated very important underscoring the value of shared decision-making as patient engagement is key in monitoring and enhancing QoL. Location of care and/or treatment, financial burden, possibility of hospitalization, potential impact on other medical conditions, and frequency and duration of treatment were of lesser concern. Participants were least concerned about the mode of administration of the treatment; none of the patients with double or triple refractory disease considered mode of administration to be fairly or very important. Those with triple refractory disease were more likely to be concerned about frequency and duration of treatment compared with other patients. Patient and care partner participants were generally aligned on priorities when making treatment decisions but had the least alignment on financial burdens with patients expressing more concern.
CONCLUSIONS
With the availability of new treatment options, patients are seeking therapies that both extend remission and OS and improve QoL, placing more importance on the need for patient engagement in the decision-making process. Treatment of MM is now often being approached from a chronic care perspective, perhaps reflective of changing attitudes about MM as a more treatable disease today.
Disclosures
Flora:Seagen Inc: Research Funding; Pfizer Inc: Research Funding. Byrd:Seagen Inc: Research Funding; Pfizer Inc: Research Funding. Platt:Bristol Myers Squibb: Research Funding; Pfizer Inc: Research Funding. Hlavacek:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Goldman:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Cappelleri:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Kennedy:Bristol Myers Squibb: Speakers Bureau; Genentech Inc: Speakers Bureau; Adaptive Biotechnologies: Consultancy; Pfizer Inc: Speakers Bureau. LeBlanc:Flatiron: Consultancy, Honoraria; CareVive: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Duke University: Research Funding; UpToDate: Patents & Royalties; Deverra Therapeutics: Research Funding; GSK: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Dosentrx: Current equity holder in private company; Meter Health: Consultancy, Honoraria; American Cancer Society: Research Funding; BlueNote: Consultancy, Honoraria; Jazz Pharmaceuticals: Research Funding; Incyte: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Speakers Bureau; Agios: Consultancy, Honoraria, Speakers Bureau; Agilix: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Leukemia and Lymphoma Society: Research Funding; National Institute of Nursing Research/National Institutes of Health: Research Funding; Seattle Genetics: Research Funding; Servier: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal